ctx clinical trial
Actual Study Start Date. The ongoing Phase 12 open-label trial CLIMB-SCD-121 is designed to assess the safety and efficacy of a single dose of CTX001 in patients ages 12 to 35 with severe SCD.
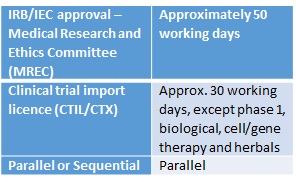
Regulatory Timelines In The Asia Pacific 乔治临床 George Clinical Cn
Estimated Primary Completion Date.
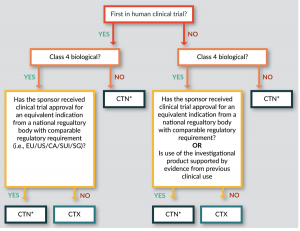
. The CTN pathway is by far the most frequent regulatory pathway in. CTX Clinical Trial Exemption An approval process. A Phase 1 Dose Escalation and Cohort Expansion Study of the Safety and Efficacy of Allogeneic CRISPR-Cas9-Engineered T Cells CTX110 in Subjects With Relapsed or Refractory B-Cell Malignancies CARBON Actual Study Start Date.
Estimated Study Completion Date. Actual Study Start Date. Adult 16 years of age and older and Pediatric under 16 years of age.
As such CTX is a cryopreserved clinical and commercial-grade cell therapy product capable of treating all eligible patients presenting. The clinical trial sponsor must also comply with the requirements of the therapeutic goods legislation as well as any other Commonwealth or stateterritory legislation in relation to clinical trials and. The patient has a diagnosis of CTX.
A Phase 1 Dose Escalation and Cohort Expansion Study of the Safety and Efficacy of Allogeneic CRISPR-Cas9-Engineered T Cells CTX130 in Subjects With Advanced Relapsed or Refractory Renal Cell Carcinoma With Clear Cell Differentiation. In the Adult group patients will switch from taking CDCA to taking a placebo. The patient has a diagnosis of cataracts with known etiology other than CTX.
Travere Therapeutics is conducting a Phase 3 clinical trial to examine the safety and efficacy of Chenodal to treat CTX. Doctors will also look at the safety and potential side effects of CDCA treatment. Under the CTA scheme as with CTX sponsors will still need to apply to.
The study has 2 groups. In the RESTORE study doctors will look at markers of CTX in the urine to see if they are lowered when CDCA is used to treat CTX. Estimated Study Completion Date.
Study to Evaluate Patients with Cerebrotendinous Xanthomatosis sponsored by Travere Therapeutics. Current CTX Clinical Trials. Join our mailing list to receive information and news as we begin to gather and expand the CTX community.
Since the last publication of Guideline for the application of Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX 5thEdition in 2009 we have witnessed robust growth in clinical research industry with the aim to achieve at least 1000 clinical trials to generate GNI of RM5784 million by the year 2020 in Malaysia. Each patient will be asked to participate in a long-term follow-up trial. The CTX Alliance is a newly-formed patient organization solely dedicated to providing resources support and promoting research for CTX patients families and healthcare providers.
The clinical trial sponsors responsibilities in relation to Good Clinical Practice GCP are set out in section 5 of the ICH Guideline for Good Clinical Practice with TGA annotations. A Phase 123 Study to Evaluate the Safety and Efficacy of a Single Dose of Autologous CRISPR-Cas9 Modified CD34 Human Hematopoietic Stem and Progenitor Cells CTX001 in Subjects With Severe Sickle Cell Disease. CX-2029-001 a Phase 12 study in patients with esophageal lung head and neck cancers or lymphoma.
To learn more about this study you or your doctor may contact the study research staff using the contacts provided below. A Phase 1 Dose Escalation and Cohort Expansion Study of the Safety and Efficacy of Anti-BCMA Allogeneic CRISPR-Cas9-Engineered T Cells CTX120 in Subjects With Relapsed or Refractory Multiple Myeloma. Doctors will also look at the safety and potential side effects of CDCA treatment.
The RESTORE study is a Phase 3 clinical trial looking at an investigational medication called chenodeoxycholic acid also called Chenodal or CDCA. CTX is a rare progressive disorder that can affect the brain spinal cord tendons eyes and arteries. Envisioned by the Texas Regional Clinical and Translational Science Award CTSA Consortium TRCC this collaboration brings together premier academic institutions physician.
Vertex Pharmaceuticals and CRISPR Therapeutics have reported positive interim results from two Phase III clinical trials of investigational ex-vivo CRISPRCas9 gene-edited therapy CTX001. Actual Study Start Date. Chenodal is not indicated for the treatment of CTX but has received a medical necessity determination in the US by the FDA.
Diagnosis of CTX Received treatment with CDCA Age between 2 and 75 years Having at least one cholestanol level andor urinary bile alcohol level no more than 3 months prior to treatment with CDCA and one cholestanol level andor urinary bile alcohol level post-treatment within 2 years from the beginning of therapy with CDCA. For general information Learn About Clinical Studies. CX-2029 is a conditionally activated antibody-drug conjugate Probody therapeutic employing the MMAE payload and targeting CD71.
The schemes previous name of CTX underscored the exemption given by the TGA to a sponsor from entering their therapeutic good in the ARTG before conducting a clinical trial. Estimated Study Completion Date. The patient has cataracts caused by cataractogenic treatments.
CTX001 involves the engineering of a patients hematopoietic stem cells to generate high foetal haemoglobin levels in red blood cells. Choosing to participate in a study is an important personal decision. In the RESTORE study doctors will look at markers of CTX in the urine to see if they are lowered when CDCA is used to treat CTX.
The Therapeutic Goods Administration TGA directly reviews the planned clinical trial and must give their approval for the clinical trial to go ahead. Estimated Primary Completion Date. Estimated Primary Completion Date.
Talk with your doctor and family members or friends about deciding to join a study. CTX has been shown to be safe and well-tolerated in a first-in-man UK clinical trial PISCES I in eleven disabled stroke patients who were followed up for at least two years post-treatment. Estimated Primary Completion Date.
We are inviting people with Cerebrotendinous Xanthomatosis CTX who may be interested in participating in a research study. The trial will enroll up to 45 patients and follow patients for approximately two years after infusion. Clinical Trials Xpress CTX is an initiative of the University of Texas System established to provide an efficient and scalable centralized operating model for conducting multi-site clinical trials.
Clinical trials conducted in Australia are subject to various regulatory controls to ensure the safety of participants. A Phase 12 clinical trial NCT03745287 called CLIMB-SCD-121 was started in November 2018 to investigate the use of CTX001 in sickle cell disease. The patient has taken or is currently taking cholic acid or chenodeoxycholic acid.
The open-label multi-site single-dose trial is recruiting 45 patients ages 18 to 35 with severe sickle cell disease in the US Canada Belgium Germany and Italy. The patient has participated in an interventional clinical trial in the. The RESTORE study is enrolling pediatric 1 month to 16 years old and.
Malaysia S Clinical Research Ecosystem

Tga Presentation Tga S Role In Clinical Trials Regulation And Admini

Malaysian Guideline For Application Of Clinical Import Licence Ctil
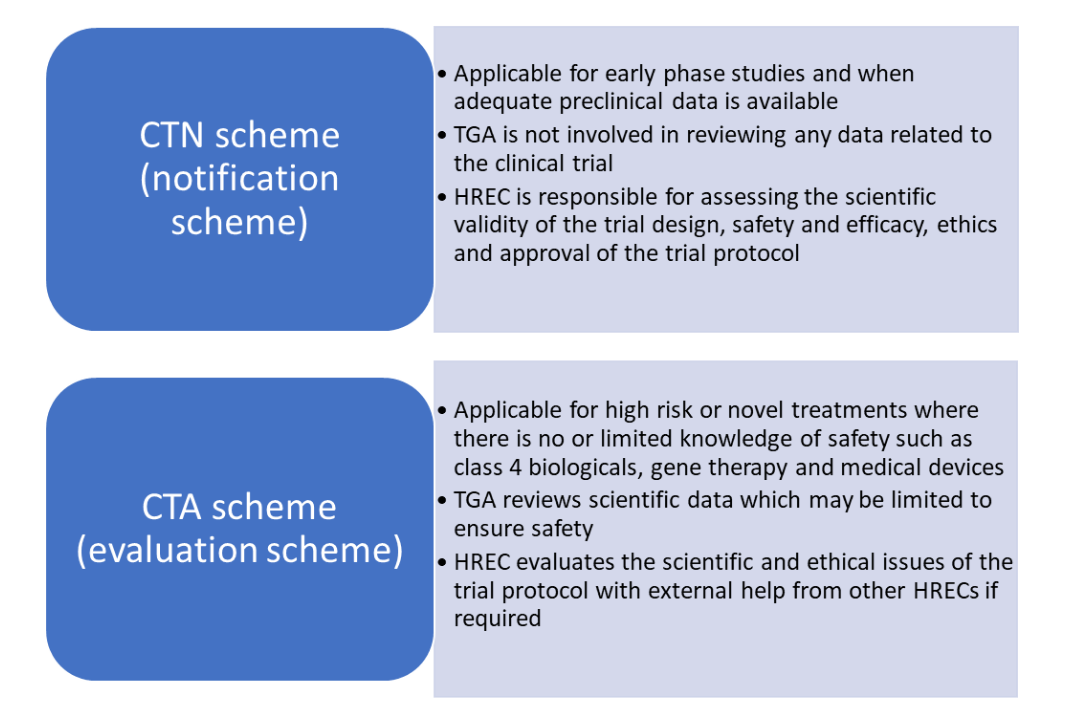
Clinical Trial Approval Process In Australia Prorelix Research

Compass Therapeutics On Twitter Compass Therapeutics Inc Otc Cmpx And Abl Bio Kosdaq 298380 Presented Clinical Trial Data For Ctx 009 Abl001 Es104 At An Oral Plenary Session During The Aacr Nci Eortc International Conference On Molecular

Bioinsights The Regulatory Environment For Cell Therapies In Australia An Opportunity To Expedite Clinical Development

Restore Study Cerebrotendinous Xanthomatosis Ctx Research Study

Thor 707 For Cancer Clinical Trial 2022 Power

Outline Of The Clinical Trial Protocol Non Small Cell Lung Cancer Download Scientific Diagram
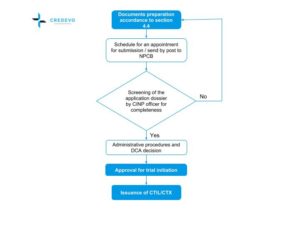
Clinical Trials In Malaysia Why And How To Start Credevo Articles

Using Australian Clinical Sites Challenges For International Sponsors Prof A J Tony Webber Clinical Network Services Pty Ltd Brisbane Australia Ppt Download

Regulatory Requirements For Clinical Trials Australia Vs The Us

Clinical Trials Medical Device Trials Genesis Research Services
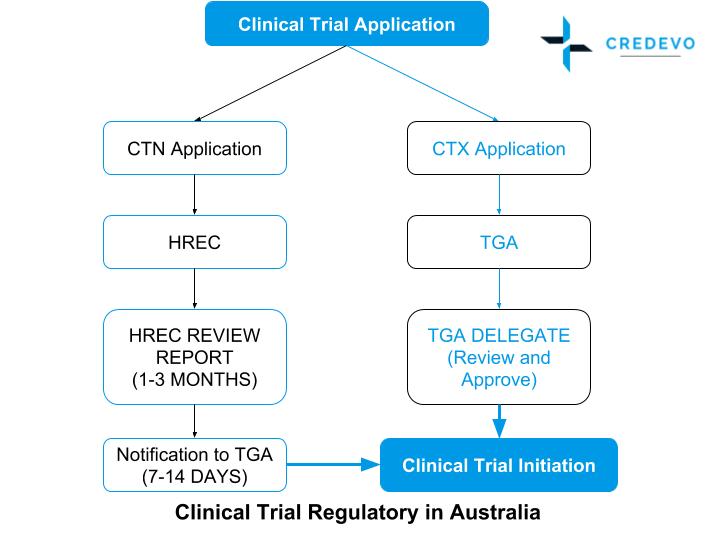
How To Get Started With Your Clinical Trials In Australia
Celtaxsys Completes Phase I Study Of Ctx 4430 In Cystic Fibrosis Patients Clinical Trials Arena

Regulatory Timelines In The Asia Pacific 乔治临床 George Clinical Cn
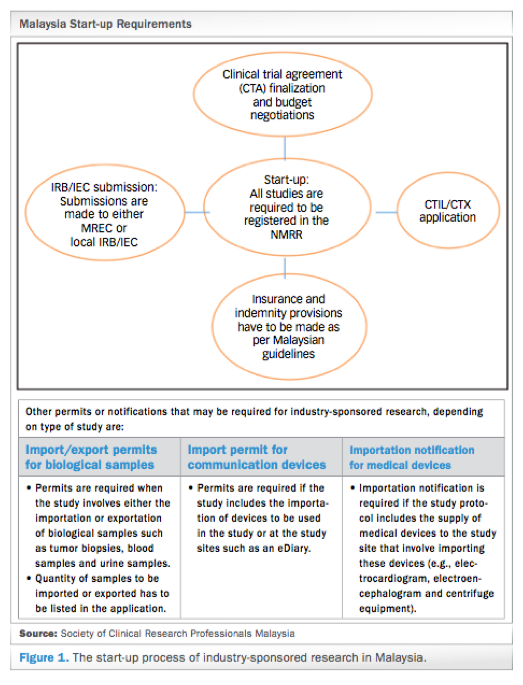
Malaysia S Clinical Research Ecosystem
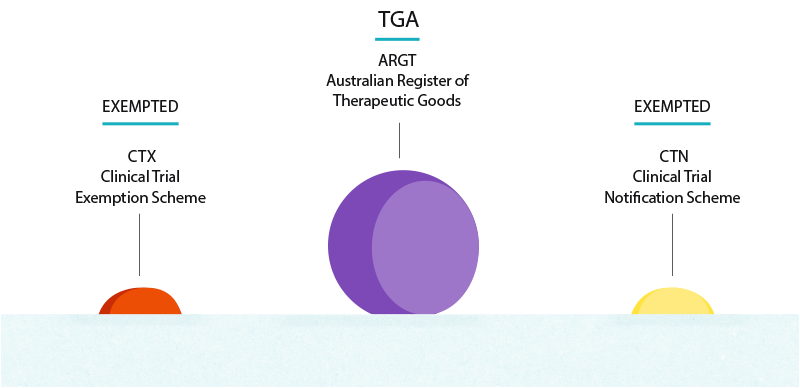
0 Response to "ctx clinical trial"
Post a Comment